Last Update: 4 Jan 2021
R Markdown:
WGCNA.Rmd
Network analysis with WGCNA
There are many gene correlation network builders but we shall provide an example of the WGCNA R Package.
The WGCNA R package builds “weighted gene correlation networks for analysis” from expression data. It was originally published in 2008 and cited as the following:
- Langfelder, P. and Horvath, S., 2008. WGCNA: an R package for weighted correlation network analysis. BMC bioinformatics, 9(1), p.559.
- Zhang, B. and Horvath, S., 2005. A general framework for weighted gene co-expression network analysis. Statistical applications in genetics and molecular biology, 4(1).
More information
- Recent PubMed Papers
- Original WGCNA tutorials - Last updated 2016
- Video: ISCB Workshop 2016 - Co-expression network analysis using RNA-Seq data (Keith Hughitt)
Installing WGCNA
We will assume you have a working R environment. If not, please see the following tutorial:
Since WGCNA is an R package, we will need to start an R environment and install from R’s package manager, CRAN.
1
2
install.packages("WGCNA") # WGCNA is available on CRAN
library(WGCNA)
Overview
The WGCNA pipeline is expecting an input matrix of RNA Sequence
counts. Usually we need to rotate (transpose) the input data so rows =
treatments and columns = gene probes.
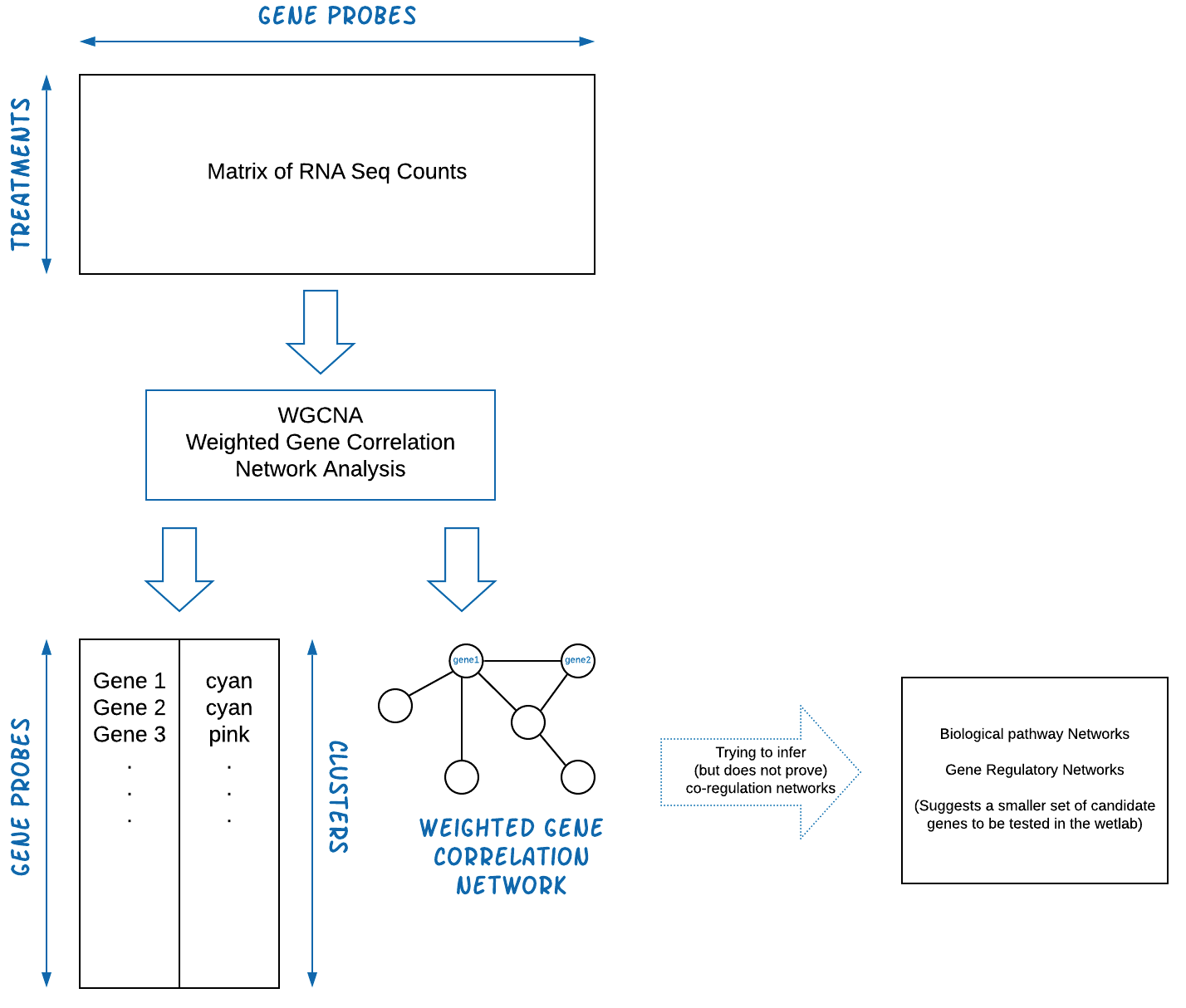
The output of WGCNA is a list of clustered genes, and weighted gene correlation network files.
Example Dataset
We shall start with an example dataset about Maize and Ligule Development. For more information, please see the following paper:
- Johnston, R., Wang, M., Sun, Q., Sylvester, A.W., Hake, S. and Scanlon, M.J., 2014. Transcriptomic analyses indicate that maize ligule development recapitulates gene expression patterns that occur during lateral organ initiation. The Plant Cell, 26(12), pp.4718-4732.
The dataset can be downloaded from the NCBI entry or using wget from
the bash commandline.
Brief Description of the experiment and data:
- The maize leaf is an ideal system to study plant morphogenesis
- It is divided into a proximal (close to stem) sheath and a distal blade each with distinct developmental patterning.
- Specialized epidermal fringe ligule and auricle structures form at the blade-sheath boundary.
For full metadata: see the NCBI GEO page for GSE61333
The SRX vs sample relation is given here broadly captured from the full metadata file:
| Sample_relation | SRR_ID | Maize_cultivar | Genotype | Sample_description | Sample_title |
|---|---|---|---|---|---|
| SRX699505 | SRR1573504 | B73 | wild-type | all cell layers Leaf blade; Repeat:3 | B-3 |
| SRX699506 | SRR1573505 | B73 | wild-type | all cell layers Leaf blade; Repeat:4 | B-4 |
| SRX699507 | SRR1573506 | B73 | wild-type | all cell layers Leaf blade; Repeat:5 | B-5 |
| SRX699508 | SRR1573507 | B73 | wild-type | all cell layers Leaf ligule; Repeat:3 | L-3 |
| SRX699509 | SRR1573508 | B73 | wild-type | all cell layers Leaf ligule; Repeat:4 | L-4 |
| SRX699510 | SRR1573509 | B73 | wild-type | all cell layers Leaf ligule; Repeat:5 | L-5 |
| SRX699511 | SRR1573510 | B73 | wild-type | all cell layers Leaf sheath; Repeat:3 | S-3 |
| SRX699512 | SRR1573511 | B73 | wild-type | all cell layers Leaf sheath; Repeat:4 | S-4 |
| SRX699513 | SRR1573512 | B73 | wild-type | all cell layers Leaf sheath; Repeat:5 | S-5 |
| SRX699514 | SRR1573513 | B73 | wild-type | Wild-type tissue from P6 leaf primordia all cell layers. Repeat 1 | wtL-1 |
| SRX699515 | SRR1573514 | B73 | wild-type | Wild-type tissue from P6 leaf primordia all cell layers. Repeat 2 | wtL-2 |
| SRX699516 | SRR1573515 | B73 | wild-type | Wild-type tissue from P6 leaf primordia all cell layers. Repeat 3 | wtL-3 |
| SRX699517 | SRR1573516 | B73 | lg1-R | lg1 mutant tissue from P6 leaf primordia all cell layers. Repeat 1 | lg1-1 |
| SRX699518 | SRR1573517 | B73 | lg1-R | lg1 mutant tissue from P6 leaf primordia all cell layers. Repeat 2 | lg1-2 |
| SRX699519 | SRR1573518 | B73 | lg1-R | lg1 mutant tissue from P6 leaf primordia all cell layers. Repeat 3 | lg1-3 |
| SRX699520 | SRR1573519 | B73 | wild-type | adaxial L1 tissue was captured. blade; Repeat:1 | B_L1.1 |
| SRX699521 | SRR1573520 | B73 | wild-type | adaxial L1 tissue was captured. blade; Repeat:2 | B_L1.2 |
| SRX699522 | SRR1573521 | B73 | wild-type | adaxial L1 tissue was captured. blade; Repeat:3 | B_L1.3 |
| SRX699523 | SRR1573522 | B73 | wild-type | adaxial L1 tissue was captured. ligule; Repeat:1 | L_L1.1 |
| SRX699524 | SRR1573523 | B73 | wild-type | adaxial L1 tissue was captured. ligule; Repeat:2 | L_L1.2 |
| SRX699525 | SRR1573524 | B73 | wild-type | adaxial L1 tissue was captured. ligule; Repeat:3 | L_L1.3 |
| SRX699526 | SRR1573525 | B73 | wild-type | adaxial L1 tissue was captured. sheath; Repeat:1 | S_L1.1 |
| SRX699527 | SRR1573526 | B73 | wild-type | adaxial L1 tissue was captured. sheath; Repeat:2 | S_L1.2 |
| SRX699528 | SRR1573527 | B73 | wild-type | adaxial L1 tissue was captured. sheath; Repeat:3 | S_L1.3 |
For more details refer to the figure below:
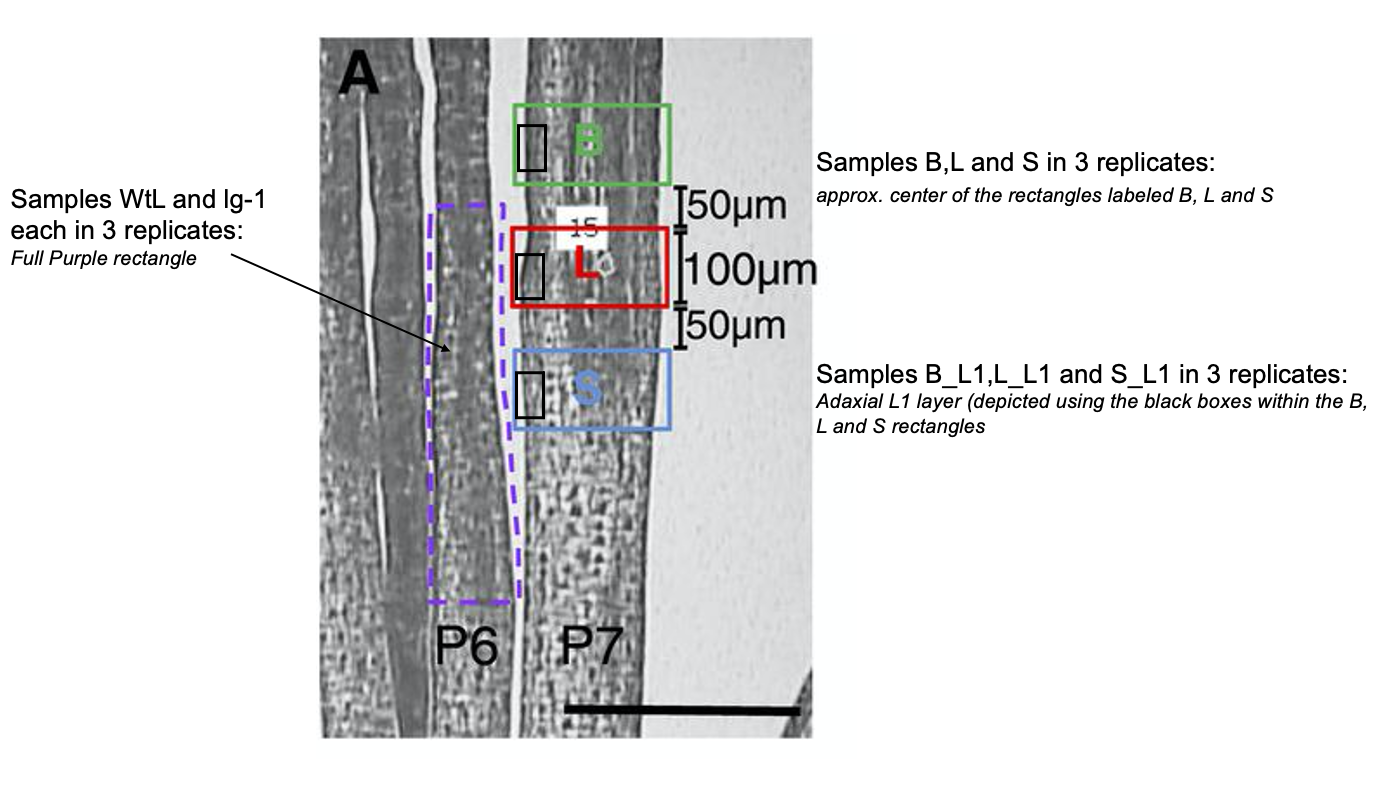
This shows how the various tissue samples were microdissected. This is modified from original Fig 2 in Johnston et al. 2014,
1
2
3
4
5
wget ftp://ftp.ncbi.nlm.nih.gov/geo/series/GSE61nnn/GSE61333/suppl/GSE61333_ligule_count.txt.gz
gunzip GSE61333_ligule_count.txt.gz
ls -ltr *.txt
#> -rw-r--r-- 1 username staff 7.5M Jan 4 09:48 GSE61333_ligule_count.txt
Load R Libraries
This analysis requires the following R libraries. You might need to install the library if it’s not already on your system.
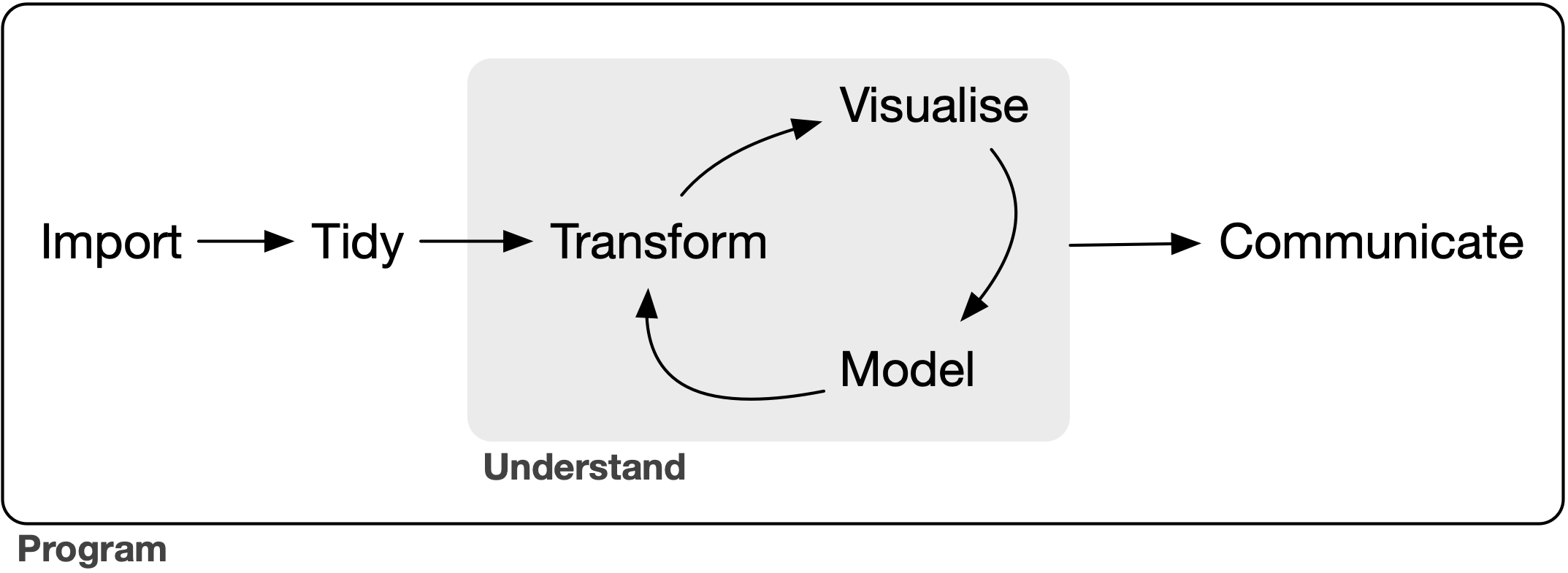
We’re going to conform to the tidyverse ecosystem. For a discussion on its benefits see “Welcome to the Tidyverse” (Wickham et al, 2019). This allows us to organize the pipeline in the following framework (Fig. from “R for Data Science” (Wickham and Grolemund, 2017)):
1
2
3
4
5
# Uncomment and modify the following to install any missing packages
# install.packages(c("tidyverse", "magrittr", "WGCNA))
library(tidyverse) # tidyverse will pull in ggplot2, readr, other useful libraries
library(magrittr) # provides the %>% operator
library(WGCNA)
Tidy the Dataset, and using exploratory graphics
Load and look at the data
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
# ==== Load and clean data
data <- readr::read_delim("data/GSE61333_ligule_count.txt", # <= path to the data file
delim = "\t")
#>
#> ── Column specification ────────────────────────────────────────────────────────
#> cols(
#> .default = col_double(),
#> Count = col_character()
#> )
#> ℹ Use `spec()` for the full column specifications.
data[1:5,1:10] # Look at first 5 rows and 10 columns
#> # A tibble: 5 x 10
#> Count `B-3` `B-4` `B-5` `L-3` `L-4` `L-5` `S-3` `S-4` `S-5`
#> <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 AC147602.5_FG004 0 0 0 0 0 0 0 0 0
#> 2 AC148152.3_FG001 0 0 0 0 0 0 0 0 0
#> 3 AC148152.3_FG002 0 0 0 0 0 0 0 0 0
#> 4 AC148152.3_FG005 0 0 0 0 0 0 0 0 0
#> 5 AC148152.3_FG006 0 0 0 0 0 0 0 0 0
# str(data) # str = structure of data, useful for debugging data type mismatch errors
names(data)[1] = "GeneId"
names(data) # Look at the column names
#> [1] "GeneId" "B-3" "B-4" "B-5" "L-3" "L-4" "L-5" "S-3"
#> [9] "S-4" "S-5" "B_L1.1" "B_L1.2" "B_L1.3" "L_L1.1" "L_L1.2" "L_L1.3"
#> [17] "S_L1.1" "S_L1.2" "S_L1.3" "wtL-1" "wtL-2" "wtL-3" "lg1-1" "lg1-2"
#> [25] "lg1-3"
If you are in RStudio, you can also click on the data object in the
Environment tab to see an Excel-like view of the data.
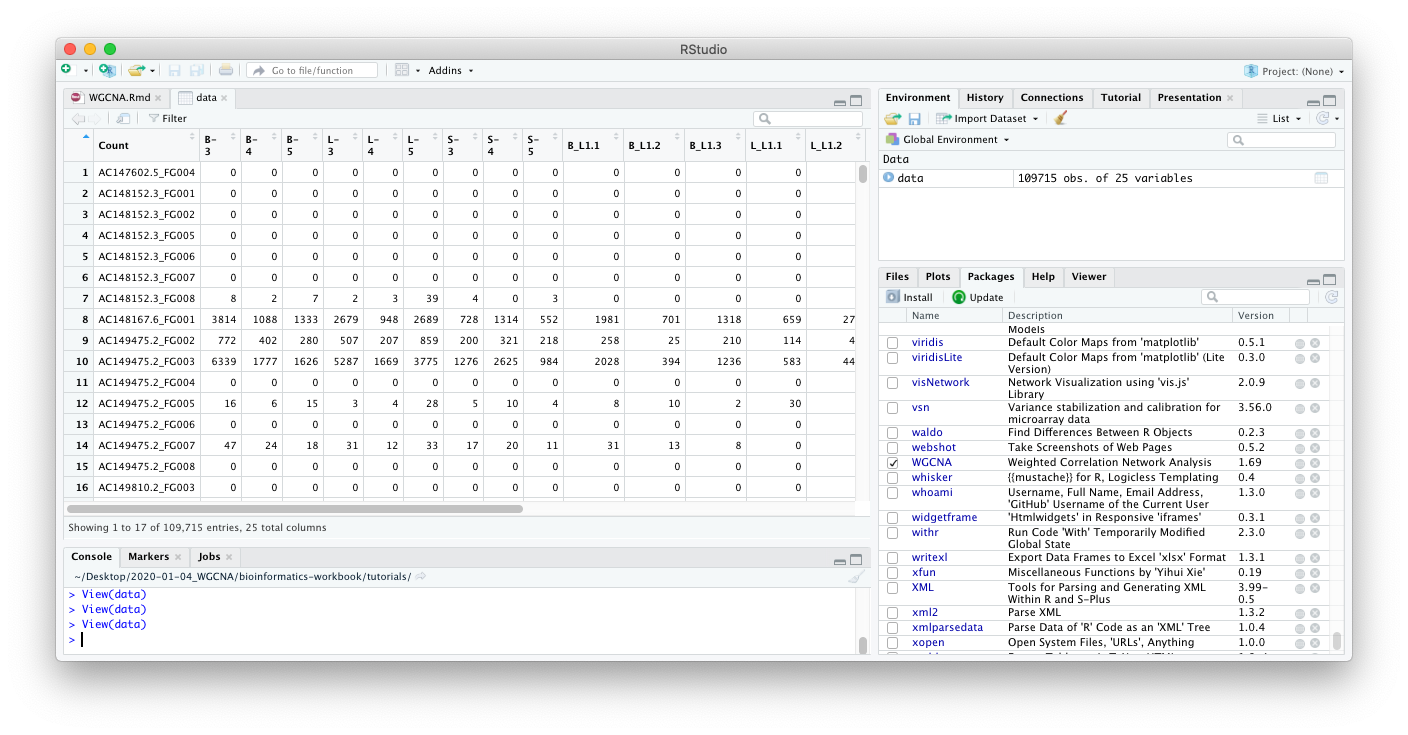
From looking at the data, we can come to the following insights:
-
We see that
rows=gene probeswhich probably meanscolumns=treatmentswhich is the opposite of what’s needed in WGCNA (rows=treatment,columns=gene probes). This dataset will need to be rotated (transposed) before sending to WGCNA. -
This is also wide data, we will convert this to tidy data before visualization. For Hadley Wickham’s tutorial on the what and why to convert to tidy data, see https://r4ds.had.co.nz/tidy-data.html.
-
The column names are prefixed with the treatment group (e.g.
B-3,B-4, andB-5are three replicates of the treatment “B”). -
TODO: Markdown table describing treatments here mean here.
e.g. B=?, S=?, L=?
The following R commands clean and tidy the dataset for exploratory graphics.
1
2
3
4
5
6
7
8
9
10
11
12
13
col_sel = names(data)[-1] # Get all but first column name
mdata <- data %>%
tidyr::pivot_longer(
., # The dot is the the input data, magrittr tutorial
col = all_of(col_sel)
) %>%
mutate(
group = gsub("-.*","", name) %>% gsub("[.].*","", .) # Get the shorter treatment names
)
# Optional step --- order the groups in the plot.
# mdata$group = factor(mdata$group,
# levels = c("B", "B_L1", ....)) #<= fill the rest of this in
Think through what kinds of plots may tell you something about the dataset. Below, we have provided an example plot to identify any obvious outliers. This may take a while to plot.
1
2
3
4
5
6
7
8
9
10
11
12
13
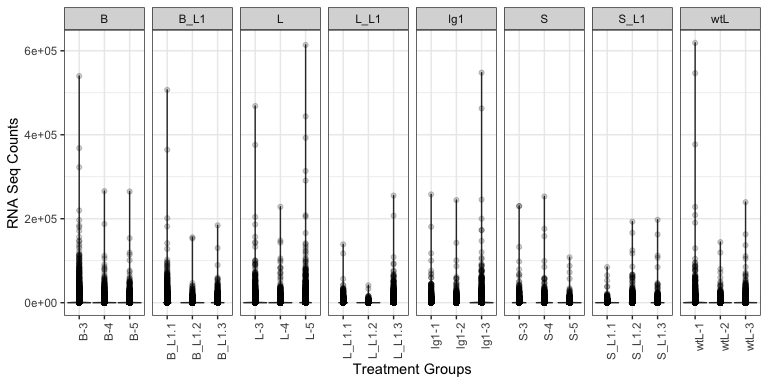
# ==== Plot groups (Sample Groups vs RNA Seq Counts) to identify outliers
(
p <- mdata %>%
ggplot(., aes(x = name, y = value)) + # x = treatment, y = RNA Seq count
geom_violin() + # violin plot, show distribution
geom_point(alpha = 0.2) + # scatter plot
theme_bw() +
theme(
axis.text.x = element_text(angle = 90) # Rotate treatment text
) +
labs(x = "Treatment Groups", y = "RNA Seq Counts") +
facet_grid(cols = vars(group), drop = TRUE, scales = "free_x") # Facet by hour
)
Normalize Counts with DESeq
We’ll use DESeq to normalize the counts before sending to WGCNA. Optionally you could subset to only genes that are differentially expressed between groups. (The smaller the number of genes, the faster WGCNA will run. Although there is some online discussion if this breaks the scale-free network assumption.)
1
library(DESeq2)
Prepare DESeq input, which is expecting a matrix of integers.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
de_input = as.matrix(data[,-1])
row.names(de_input) = data$GeneId
de_input[1:5,1:10]
#> B-3 B-4 B-5 L-3 L-4 L-5 S-3 S-4 S-5 B_L1.1
#> AC147602.5_FG004 0 0 0 0 0 0 0 0 0 0
#> AC148152.3_FG001 0 0 0 0 0 0 0 0 0 0
#> AC148152.3_FG002 0 0 0 0 0 0 0 0 0 0
#> AC148152.3_FG005 0 0 0 0 0 0 0 0 0 0
#> AC148152.3_FG006 0 0 0 0 0 0 0 0 0 0
#str(de_input)
meta_df <- data.frame( Sample = names(data[-1])) %>%
mutate(
Type = gsub("-.*","", Sample) %>% gsub("[.].*","", .)
)
dds <- DESeqDataSetFromMatrix(round(de_input),
meta_df,
design = ~Type)
#> converting counts to integer mode
#> Warning in DESeqDataSet(se, design = design, ignoreRank): some variables in
#> design formula are characters, converting to factors
dds <- DESeq(dds)
#> estimating size factors
#> estimating dispersions
#> gene-wise dispersion estimates
#> mean-dispersion relationship
#> final dispersion estimates
#> fitting model and testing
vsd <- varianceStabilizingTransformation(dds)
library(genefilter) # <= why is this here?
#>
#> Attaching package: 'genefilter'
#> The following objects are masked from 'package:matrixStats':
#>
#> rowSds, rowVars
#> The following object is masked from 'package:readr':
#>
#> spec
wpn_vsd <- getVarianceStabilizedData(dds)
rv_wpn <- rowVars(wpn_vsd)
summary(rv_wpn)
#> Min. 1st Qu. Median Mean 3rd Qu. Max.
#> 0.00000 0.00000 0.00000 0.08044 0.03322 11.14529
q75_wpn <- quantile( rowVars(wpn_vsd), .75) # <= original
q95_wpn <- quantile( rowVars(wpn_vsd), .95) # <= changed to 95 quantile to reduce dataset
expr_normalized <- wpn_vsd[ rv_wpn > q95_wpn, ]
expr_normalized[1:5,1:10]
#> B-3 B-4 B-5 L-3 L-4 L-5 S-3
#> AC149818.2_FG001 7.600901 7.077399 7.803434 7.220840 7.410408 8.028223 7.160846
#> AC149829.2_FG003 8.782014 8.179876 7.900062 8.299778 7.529891 8.631731 8.055118
#> AC182617.3_FG001 8.047244 7.120668 6.885533 7.501391 7.279413 7.809565 7.184253
#> AC186512.3_FG001 6.901539 7.389644 6.975945 6.859593 7.370816 6.633722 7.798843
#> AC186512.3_FG007 7.919688 7.754506 7.670946 7.417760 7.988427 7.904850 7.484542
#> S-4 S-5 B_L1.1
#> AC149818.2_FG001 7.401382 7.345322 6.524435
#> AC149829.2_FG003 8.744502 8.142909 8.240407
#> AC182617.3_FG001 8.140134 6.972400 7.777347
#> AC186512.3_FG001 6.949501 6.952659 6.059033
#> AC186512.3_FG007 8.375664 7.762799 6.335663
dim(expr_normalized)
#> [1] 5486 24
expr_normalized_df <- data.frame(expr_normalized) %>%
mutate(
Gene_id = row.names(expr_normalized)
) %>%
pivot_longer(-Gene_id)
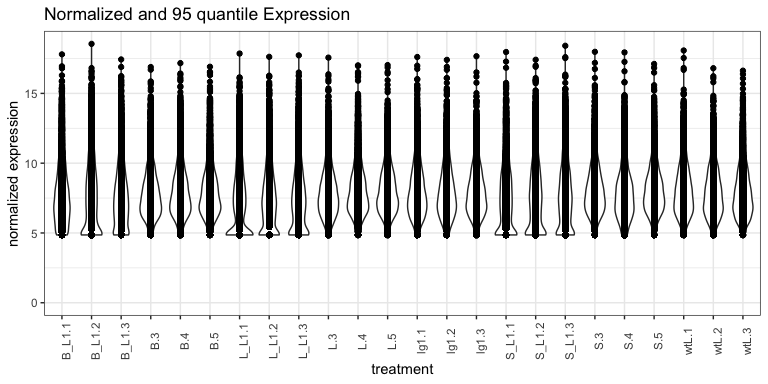
expr_normalized_df %>% ggplot(., aes(x = name, y = value)) +
geom_violin() +
geom_point() +
theme_bw() +
theme(
axis.text.x = element_text( angle = 90)
) +
ylim(0, NA) +
labs(
title = "Normalized and 95 quantile Expression",
x = "treatment",
y = "normalized expression"
)
WGCNA
Now let’s transpose the data and prepare the dataset for WGCNA.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
input_mat = t(expr_normalized)
input_mat[1:5,1:10] # Look at first 5 rows and 10 columns
#> AC149818.2_FG001 AC149829.2_FG003 AC182617.3_FG001 AC186512.3_FG001
#> B-3 7.600901 8.782014 8.047244 6.901539
#> B-4 7.077399 8.179876 7.120668 7.389644
#> B-5 7.803434 7.900062 6.885533 6.975945
#> L-3 7.220840 8.299778 7.501391 6.859593
#> L-4 7.410408 7.529891 7.279413 7.370816
#> AC186512.3_FG007 AC189795.3_FG001 AC190609.3_FG002 AC190623.3_FG001
#> B-3 7.919688 8.149041 12.64301 6.575155
#> B-4 7.754506 8.077571 11.99816 7.170788
#> B-5 7.670946 7.524430 12.12500 7.438024
#> L-3 7.417760 8.420552 12.36979 8.223261
#> L-4 7.988427 7.105196 11.64515 8.008850
#> AC192451.3_FG001 AC195340.3_FG001
#> B-3 6.700385 9.104258
#> B-4 7.325447 9.135480
#> B-5 7.819142 9.023856
#> L-3 8.052019 8.908933
#> L-4 8.528875 8.583982
We can see now that the rows = treatments and columns =
gene probes. We’re ready to start WGCNA. A correlation network will be
a complete network (all genes are connected to all other genes). Ergo we
will need to pick a threshhold value (if correlation is below threshold,
remove the edge). We assume the true biological network follows a
scale-free structure (see papers by Albert
Barabasi).
To do that, WGCNA will try a range of soft thresholds and create a diagnostic plot. This step will take several minutes so feel free to run and get coffee.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
#library(WGCNA)
allowWGCNAThreads() # allow multi-threading (optional)
#> Allowing multi-threading with up to 4 threads.
# Choose a set of soft-thresholding powers
powers = c(c(1:10), seq(from = 12, to = 20, by = 2))
# Call the network topology analysis function
sft = pickSoftThreshold(
input_mat, # <= Input data
#blockSize = 30,
powerVector = powers,
verbose = 5
)
#> pickSoftThreshold: will use block size 5486.
#> pickSoftThreshold: calculating connectivity for given powers...
#> ..working on genes 1 through 5486 of 5486
#> Power SFT.R.sq slope truncated.R.sq mean.k. median.k. max.k.
#> 1 1 0.5350 2.500 0.960 1940.0 1950.0 2840
#> 2 2 0.0642 0.331 0.897 964.0 927.0 1860
#> 3 3 0.1680 -0.444 0.859 560.0 505.0 1340
#> 4 4 0.5050 -0.822 0.906 358.0 300.0 1030
#> 5 5 0.6800 -1.070 0.935 243.0 189.0 819
#> 6 6 0.7770 -1.230 0.954 173.0 125.0 673
#> 7 7 0.8330 -1.310 0.972 127.0 85.3 564
#> 8 8 0.8660 -1.390 0.980 96.4 60.2 484
#> 9 9 0.8810 -1.450 0.981 74.8 43.2 422
#> 10 10 0.8940 -1.490 0.984 59.1 31.7 371
#> 11 12 0.9070 -1.540 0.988 38.7 17.6 295
#> 12 14 0.9150 -1.580 0.988 26.7 10.3 240
#> 13 16 0.9220 -1.570 0.985 19.1 6.3 200
#> 14 18 0.9200 -1.570 0.979 14.1 4.0 169
#> 15 20 0.9240 -1.570 0.982 10.7 2.6 145
par(mfrow = c(1,2));
cex1 = 0.9;
plot(sft$fitIndices[, 1],
-sign(sft$fitIndices[, 3]) * sft$fitIndices[, 2],
xlab = "Soft Threshold (power)",
ylab = "Scale Free Topology Model Fit, signed R^2",
main = paste("Scale independence")
)
text(sft$fitIndices[, 1],
-sign(sft$fitIndices[, 3]) * sft$fitIndices[, 2],
labels = powers, cex = cex1, col = "red"
)
abline(h = 0.90, col = "red")
plot(sft$fitIndices[, 1],
sft$fitIndices[, 5],
xlab = "Soft Threshold (power)",
ylab = "Mean Connectivity",
type = "n",
main = paste("Mean connectivity")
)
text(sft$fitIndices[, 1],
sft$fitIndices[, 5],
labels = powers,
cex = cex1, col = "red")
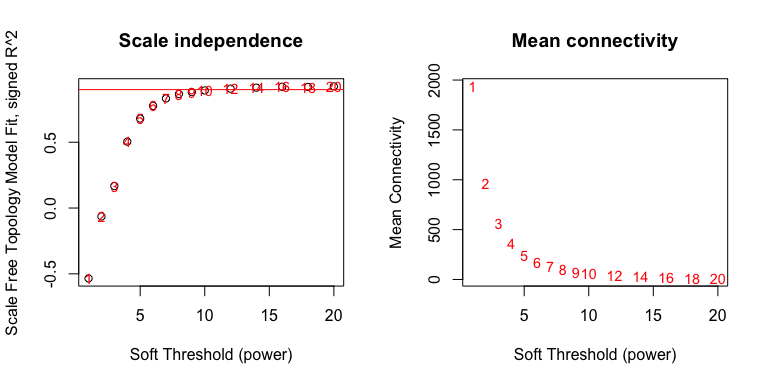
Pick a soft threshold power near the curve of the plot, so here we could
pick 7, 8 or 9. We’ll pick 9 but feel free to experiment with other
powers to see how it affects your results. Now we can create the network
using the blockwiseModules command. The blockwiseModule may take a
while to run, since it is constructing the TOM (topological overlap
matrix) and several other steps. While it runs, take a look at the
blockwiseModule documentation (link to
vignette)
for more information on the parameters. How might you change the
parameters to get more or less modules?
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
picked_power = 9
temp_cor <- cor
cor <- WGCNA::cor # Force it to use WGCNA cor function (fix a namespace conflict issue)
netwk <- blockwiseModules(input_mat, # <= input here
# == Adjacency Function ==
power = picked_power, # <= power here
networkType = "signed",
# == Tree and Block Options ==
deepSplit = 2,
pamRespectsDendro = F,
# detectCutHeight = 0.75,
minModuleSize = 30,
maxBlockSize = 4000,
# == Module Adjustments ==
reassignThreshold = 0,
mergeCutHeight = 0.25,
# == TOM == Archive the run results in TOM file (saves time)
saveTOMs = T,
saveTOMFileBase = "ER",
# == Output Options
numericLabels = T,
verbose = 3)
#> Calculating module eigengenes block-wise from all genes
#> Flagging genes and samples with too many missing values...
#> ..step 1
#> ....pre-clustering genes to determine blocks..
#> Projective K-means:
#> ..k-means clustering..
#> ..merging smaller clusters...
#> Block sizes:
#> gBlocks
#> 1 2
#> 3489 1997
#> ..Working on block 1 .
#> TOM calculation: adjacency..
#> ..will use 4 parallel threads.
#> Fraction of slow calculations: 0.000000
#> ..connectivity..
#> ..matrix multiplication (system BLAS)..
#> ..normalization..
#> ..done.
#> ..saving TOM for block 1 into file ER-block.1.RData
#> ....clustering..
#> ....detecting modules..
#> ....calculating module eigengenes..
#> ....checking kME in modules..
#> ..removing 31 genes from module 1 because their KME is too low.
#> ..removing 27 genes from module 2 because their KME is too low.
#> ..removing 1 genes from module 3 because their KME is too low.
#> ..removing 1 genes from module 5 because their KME is too low.
#> ..removing 1 genes from module 6 because their KME is too low.
#> ..removing 1 genes from module 9 because their KME is too low.
#> ..Working on block 2 .
#> TOM calculation: adjacency..
#> ..will use 4 parallel threads.
#> Fraction of slow calculations: 0.000000
#> ..connectivity..
#> ..matrix multiplication (system BLAS)..
#> ..normalization..
#> ..done.
#> ..saving TOM for block 2 into file ER-block.2.RData
#> ....clustering..
#> ....detecting modules..
#> ....calculating module eigengenes..
#> ....checking kME in modules..
#> ..removing 25 genes from module 1 because their KME is too low.
#> ..removing 5 genes from module 2 because their KME is too low.
#> ..removing 3 genes from module 3 because their KME is too low.
#> ..removing 2 genes from module 4 because their KME is too low.
#> ..removing 3 genes from module 5 because their KME is too low.
#> ..removing 1 genes from module 8 because their KME is too low.
#> ..merging modules that are too close..
#> mergeCloseModules: Merging modules whose distance is less than 0.25
#> Calculating new MEs...
cor <- temp_cor # Return cor function to original namespace
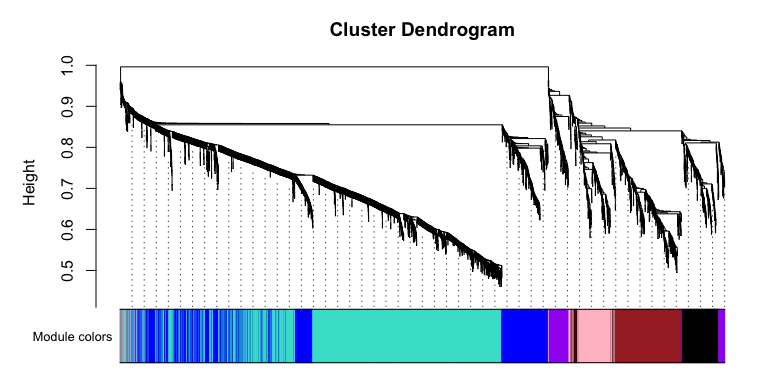
Let’s take a look at the modules, there
1
2
3
4
5
6
7
8
9
10
11
# Convert labels to colors for plotting
mergedColors = labels2colors(netwk$colors)
# Plot the dendrogram and the module colors underneath
plotDendroAndColors(
netwk$dendrograms[[1]],
mergedColors[netwk$blockGenes[[1]]],
"Module colors",
dendroLabels = FALSE,
hang = 0.03,
addGuide = TRUE,
guideHang = 0.05 )
1
2
# netwk$colors[netwk$blockGenes[[1]]]
# table(netwk$colors)
Relate Module (cluster) Assignments to Treatment Groups
We can pull out the list of modules
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
module_df <- data.frame(
gene_id = names(netwk$colors),
colors = labels2colors(netwk$colors)
)
module_df[1:5,]
#> gene_id colors
#> 1 AC149818.2_FG001 blue
#> 2 AC149829.2_FG003 blue
#> 3 AC182617.3_FG001 blue
#> 4 AC186512.3_FG001 turquoise
#> 5 AC186512.3_FG007 turquoise
write_delim(module_df,
file = "gene_modules.txt",
delim = "\t")
We have written out a tab delimited file listing the genes and their modules. However, we need to figure out which modules are associated with each trait/treatment group. WGCNA will calcuate an Eigangene (hypothetical central gene) for each module, so it easier to determine if modules are associated with different treatments.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
# Get Module Eigengenes per cluster
MEs0 <- moduleEigengenes(input_mat, mergedColors)$eigengenes
# Reorder modules so similar modules are next to each other
MEs0 <- orderMEs(MEs0)
module_order = names(MEs0) %>% gsub("ME","", .)
# Add treatment names
MEs0$treatment = row.names(MEs0)
# tidy & plot data
mME = MEs0 %>%
pivot_longer(-treatment) %>%
mutate(
name = gsub("ME", "", name),
name = factor(name, levels = module_order)
)
mME %>% ggplot(., aes(x=treatment, y=name, fill=value)) +
geom_tile() +
theme_bw() +
scale_fill_gradient2(
low = "blue",
high = "red",
mid = "white",
midpoint = 0,
limit = c(-1,1)) +
theme(axis.text.x = element_text(angle=90)) +
labs(title = "Module-trait Relationships", y = "Modules", fill="corr")
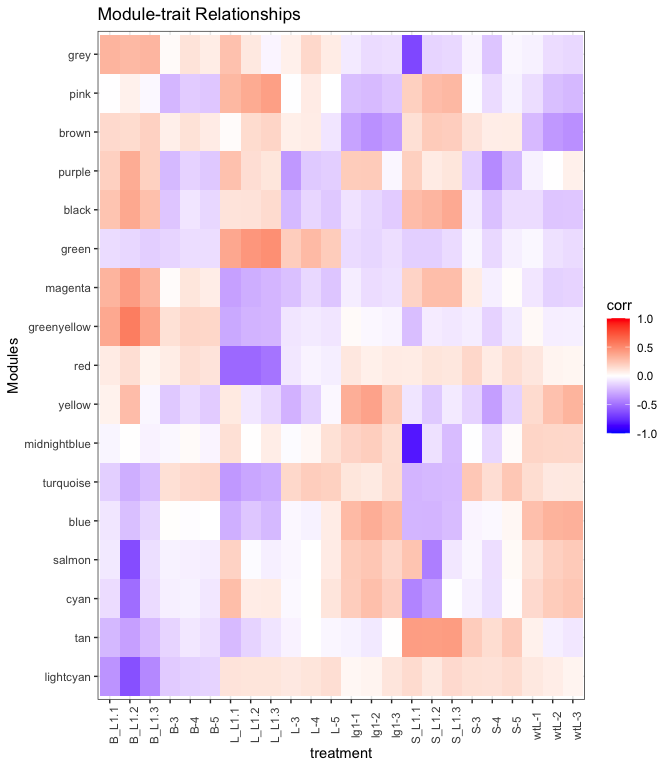
Looking at the heatmap, the green seems positively associated (red
shading) with the L groups, turquoise module is positive for the L
but negative (blue shading) for the L_L1 groups, and tan module is
positive in the S groups but negative elsewhere. There are other
patterns, think about what the patterns mean. For this tutorial we will
focus on the green, turquoise, and tan module genes.
Examine Expression Profiles
We’ll pick out a few modules of interest, and plot their expression profiles
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
# pick out a few modules of interest here
modules_of_interest = c("green", "turquoise", "tan")
# Pull out list of genes in that module
submod = module_df %>%
subset(colors %in% modules_of_interest)
row.names(module_df) = module_df$gene_id
# Get normalized expression for those genes
expr_normalized[1:5,1:10]
#> B-3 B-4 B-5 L-3 L-4 L-5 S-3
#> AC149818.2_FG001 7.600901 7.077399 7.803434 7.220840 7.410408 8.028223 7.160846
#> AC149829.2_FG003 8.782014 8.179876 7.900062 8.299778 7.529891 8.631731 8.055118
#> AC182617.3_FG001 8.047244 7.120668 6.885533 7.501391 7.279413 7.809565 7.184253
#> AC186512.3_FG001 6.901539 7.389644 6.975945 6.859593 7.370816 6.633722 7.798843
#> AC186512.3_FG007 7.919688 7.754506 7.670946 7.417760 7.988427 7.904850 7.484542
#> S-4 S-5 B_L1.1
#> AC149818.2_FG001 7.401382 7.345322 6.524435
#> AC149829.2_FG003 8.744502 8.142909 8.240407
#> AC182617.3_FG001 8.140134 6.972400 7.777347
#> AC186512.3_FG001 6.949501 6.952659 6.059033
#> AC186512.3_FG007 8.375664 7.762799 6.335663
subexpr = expr_normalized[submod$gene_id,]
submod_df = data.frame(subexpr) %>%
mutate(
gene_id = row.names(.)
) %>%
pivot_longer(-gene_id) %>%
mutate(
module = module_df[gene_id,]$colors
)
submod_df %>% ggplot(., aes(x=name, y=value, group=gene_id)) +
geom_line(aes(color = module),
alpha = 0.2) +
theme_bw() +
theme(
axis.text.x = element_text(angle = 90)
) +
facet_grid(rows = vars(module)) +
labs(x = "treatment",
y = "normalized expression")
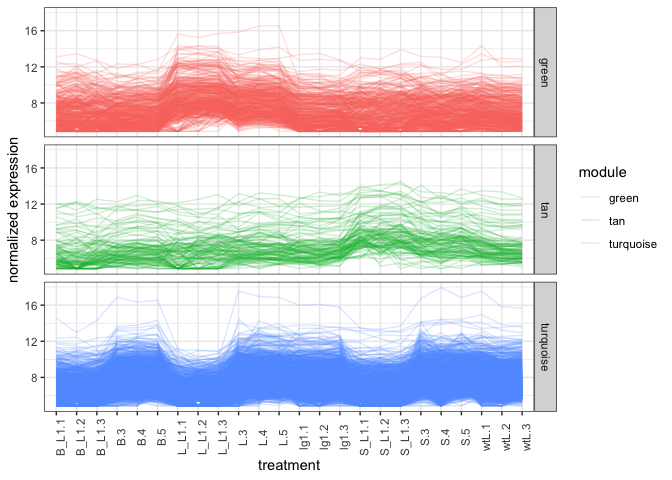
It is possible to plot the modules in green, tan, and turquoise colors
but for now we’ll use the auto colors generated by ggplot2. From the
expression line graph, the line graph matches the correlation graph
where Green module genes are higher in the L groups. Tan genes
seem higher in the S groups, and Turquoise genes are low at L_L1
and S_L1 and maybe B_L1 groups.
Generate and Export Networks
The network file can be generated for Cytoscape or as an edge/vertices file.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
genes_of_interest = module_df %>%
subset(colors %in% modules_of_interest)
expr_of_interest = expr_normalized[genes_of_interest$gene_id,]
expr_of_interest[1:5,1:5]
#> B-3 B-4 B-5 L-3 L-4
#> AC186512.3_FG001 6.901539 7.389644 6.975945 6.859593 7.370816
#> AC186512.3_FG007 7.919688 7.754506 7.670946 7.417760 7.988427
#> AC190623.3_FG001 6.575155 7.170788 7.438024 8.223261 8.008850
#> AC196475.3_FG004 6.054319 6.439899 6.424540 5.815344 6.565299
#> AC196475.3_FG005 6.194406 5.872273 6.207174 6.499828 6.314952
# Only recalculate TOM for modules of interest (faster, altho there's some online discussion if this will be slightly off)
TOM = TOMsimilarityFromExpr(t(expr_of_interest),
power = picked_power)
#> TOM calculation: adjacency..
#> ..will use 4 parallel threads.
#> Fraction of slow calculations: 0.000000
#> ..connectivity..
#> ..matrix multiplication (system BLAS)..
#> ..normalization..
#> ..done.
# Add gene names to row and columns
row.names(TOM) = row.names(expr_of_interest)
colnames(TOM) = row.names(expr_of_interest)
edge_list = data.frame(TOM) %>%
mutate(
gene1 = row.names(.)
) %>%
pivot_longer(-gene1) %>%
dplyr::rename(gene2 = name, correlation = value) %>%
unique() %>%
subset(!(gene1==gene2)) %>%
mutate(
module1 = module_df[gene1,]$colors,
module2 = module_df[gene2,]$colors
)
head(edge_list)
#> # A tibble: 6 x 5
#> gene1 gene2 correlation module1 module2
#> <chr> <chr> <dbl> <chr> <chr>
#> 1 AC186512.3_FG001 AC186512.3_FG007 0.0238 turquoise turquoise
#> 2 AC186512.3_FG001 AC190623.3_FG001 0.0719 turquoise turquoise
#> 3 AC186512.3_FG001 AC196475.3_FG004 0.143 turquoise turquoise
#> 4 AC186512.3_FG001 AC196475.3_FG005 0.0117 turquoise turquoise
#> 5 AC186512.3_FG001 AC196489.3_FG002 0.0181 turquoise turquoise
#> 6 AC186512.3_FG001 AC198481.3_FG004 0.0240 turquoise turquoise
# Export Network file to be read into Cytoscape, VisANT, etc
write_delim(edge_list,
file = "edgelist.tsv",
delim = "\t")
The edgelist.txt exported is the complete correlation network for
modules green, tan, and turquoise. The network still needs to be
subsetted down (by weight or minimal spanning) to identify hub genes.
Steps forward include identifying hub genes and gene ontology
enrichment. The edgelist can be loaded into igraph (R package) or
Cytoscape (standalone Java Application) for further network analysis.
In Summary
We have taken in RNASeq count information, identified the top (95% quantile) differentially expressed genes, sent it to WGCNA to identify modules and create the gene correlation network for those modules.
Input:
GSE61333_ligule_count.txt- RNASeq counts
Output:
gene_modules.txt- Lists genes and their WGCNA modulesedge_list.tsv- Gene correlation network for modules of interest

